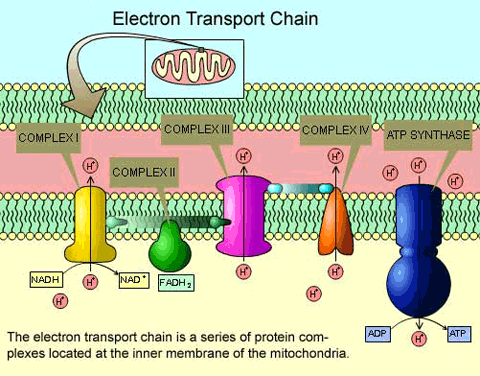
Most of the ATP originated during the aerobic catabolism of glucose does not come from the two previous routes (Glycolysis and Krebs cycle), but rather from the movement of electrons through a series of electron carriers that undergo redox reactions, which causes the accumulation of hydrogen ions in the mitochondrial matrix.
As a consequence, a concentration gradient is formed in which hydrogen ions diffuse out of the mitochondrial matrix by passing through it with the help of the complex called ATP synthase[1]. The current of hydrogen ions promotes the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP.
Electron transport chain
The electron transport chain is the last component of aerobic respiration and the last stage of glucose metabolism that uses atmospheric oxygen.
This oxygen is used as a final receptor for the electrons that have been removed from the intermediate compounds in glucose catabolism.
This oxygen is used as a final receptor for the electrons that have been removed from the intermediate compounds in glucose catabolism.
The electron transport chain consists of four large multiprotein complexes (called complexes I-IV) embedded in the inner mitochondrial membrane and two small electron transporters that move the electrons between them.
Electrons participate in a series of redox reactions in which free energy is used to shuttle hydrogen ions across the mitochondrial membrane using the ATP synthase enzyme. This process contributes to the gradient used in chemiosmosis (a mechanism based on the diffusion of ions across a membrane) that produces 90% of ATP created during aerobic glucose metabolism.
The electrons that take part in the transport chain lose their energy gradually, so to complete it, the contribution of high-energy electrons from the NADH or FADH2 compounds is necessary (generated in the previous process (Krebs cycle).
At the end of this metabolic pathway, electrons reduce oxygen molecules to oxygen ions and the extra electrons of these ions attract hydrogen ions (protons) from the surrounding environment, resulting in the release of water molecules and ATP as the final products of the electron transport chain.
The number of molecules created varies depending on a number of factors such as the number of hydrogen ions that the complexes of the electron chain pump across the mitochondrial membrane, the transport of electrons or the use of intermediate compounds produced in these routes for other purposes.
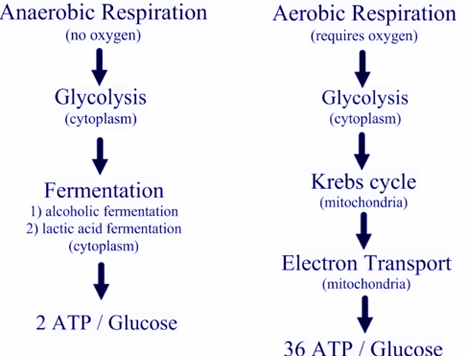
Metabolism without oxygen
In aerobic respiration, the final receptor of electrons is the oxygen molecule (O2) and ATP is produced with the assistance of high-energy electrons transported to the transport chain by the molecules NADH or FADH2.
If aerobic respiration does not take place, the NADH compound must be re-oxidased to NAD+ so that it can be reused as an electron carrier, and in this way the glycolytic pathway continues.
Living organisms employ two different mechanisms to achieve this:
Living organisms employ two different mechanisms to achieve this:
▣ They can use an organic molecule as the final acceptor of electrons to regenerate NAD+ from NADH in a process called fermentation.
One of the most well known fermentation processes is lactic acid fermentation, used by red blood cells in skeletal muscles of mammals when they lack enough oxygen to continue aerobic respiration.
One of the most well known fermentation processes is lactic acid fermentation, used by red blood cells in skeletal muscles of mammals when they lack enough oxygen to continue aerobic respiration.
▣ The second option is to use an inorganic molecule instead.
In both, organisms transform energy to use it in the absence of oxygen and they are known as anaerobic cellular respiration.Regulation of cellular respiration
Cellular respiration must be regulated in order to supply the required amounts of energy at any time in the form of ATP.
The cell must regulate, therefore, its metabolism and for that have a broad variety of mechanisms.
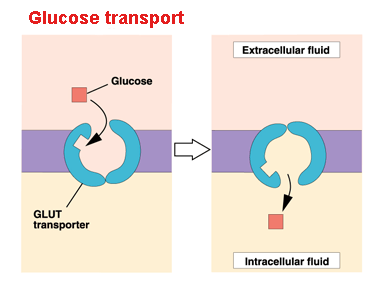
For instance, glucose entering the cell via the plasma membrane is controlled by transport proteins (GLUT proteins). But most of the control of the respiratory process is performed by specific enzymes that act on each route.
These enzymes react to the available levels of the nucleosides ATP, ADP, AMP, NAD+ and FAD, which, in turn, increase or decrease enzyme activity on the routes where they participate.
The cell must regulate, therefore, its metabolism and for that have a broad variety of mechanisms.
For instance, glucose entering the cell via the plasma membrane is controlled by transport proteins (GLUT proteins). But most of the control of the respiratory process is performed by specific enzymes that act on each route.
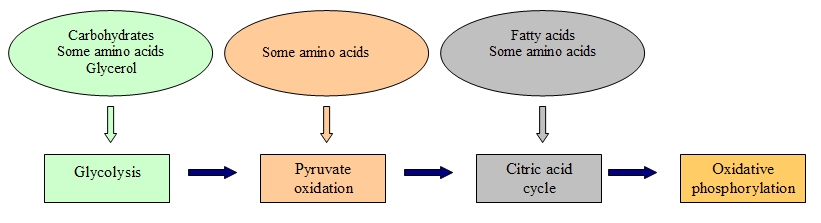
We have seen that glucose metabolism is responsible for providing energy to living cells. Yet, living beings consume a broad range of nutrients other than glucose in their diets. Hence, how do these foods become ATP in our cells?
Eventually the catabolic pathways of lipids, proteins and carbohydrates are connected with glycolysis and citric acid cycle pathways.
These pathways are not closed cycles, but many of their substrates, intermediate and final products are used in other routes.
Eventually the catabolic pathways of lipids, proteins and carbohydrates are connected with glycolysis and citric acid cycle pathways.
These pathways are not closed cycles, but many of their substrates, intermediate and final products are used in other routes.
[1] It is a transmembrane protein complex (enzyme) that catalyses ATP synthesis by the supplied energy from a proton flow (H+) and by adding a phosphate group to ADP.
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
https://chelseaharripersad.wordpress.com/2013/04/13/the-electron-transport-chain/
http://healthylifemed.com/aerobic-vs-anaerobic-respiration/
https://adapaproject.org/bbk_temp/tiki-index.php?page=Leaf%3A+Why+do+cells+need+fermentation+to+continue+glycolysis%3F
http://163.178.103.176/Fisiologia/general/dinamica/FG05_17a.jpg
https://chelseaharripersad.wordpress.com/2013/04/13/the-electron-transport-chain/
http://healthylifemed.com/aerobic-vs-anaerobic-respiration/
https://adapaproject.org/bbk_temp/tiki-index.php?page=Leaf%3A+Why+do+cells+need+fermentation+to+continue+glycolysis%3F
http://163.178.103.176/Fisiologia/general/dinamica/FG05_17a.jpg






Your opinion matters