Currently, there are two basic problems related to drug dosage.
The first when the drug is too soluble in water and a controlled release system is required to counteract this. The second when the drug, on the contrary, has no or very low solubility, and therefore, it does not dissolve in water.
The first when the drug is too soluble in water and a controlled release system is required to counteract this. The second when the drug, on the contrary, has no or very low solubility, and therefore, it does not dissolve in water.
This second problem is vital to solve because, today, it is estimated that between 75 and 80% of new chemical substances that come out of academic laboratories and pharmaceutical companies are not water-soluble.
This challenge is to get the main constituents of the drug to dissolve in the gastrointestinal tract, and, in this way, to perform their systematic action[1].
This challenge is to get the main constituents of the drug to dissolve in the gastrointestinal tract, and, in this way, to perform their systematic action[1].
Thanks to nanoparticle engineering technologies, new crystalline drugs can be designed with a really small size (between 200 and 300 nm), which enables them to have faster dissolution rates than larger particles.
Once they are manufactured, these nanoparticles are incorporated into the different drug delivery systems where they are dissolved. In this way the drug is available to be absorbed by the lungs or the digestive system, depending on which route of administration has been chosen.
An alternative would be a drug  dissolved in an organic solvent (not water) such as acetonitrile, methanol or ethanol. The drug is then dissolved with some kind of stabiliser in order to then be rapidly frozen. As a result of this procedure we obtain non-crystalline pharmaceutical substances, named amorphous forms or amorphous morphology, which can be dissolved at much higher concentrations than their corresponding (water) crystalline forms.
dissolved in an organic solvent (not water) such as acetonitrile, methanol or ethanol. The drug is then dissolved with some kind of stabiliser in order to then be rapidly frozen. As a result of this procedure we obtain non-crystalline pharmaceutical substances, named amorphous forms or amorphous morphology, which can be dissolved at much higher concentrations than their corresponding (water) crystalline forms.
Thus ‘super-saturation’ of an aqueous solution is achieved, which facilitates the drug’s dissolution at high concentrations in the stomach and in the upper small intestine to be subsequently absorbed and distributed through the whole organism.
 dissolved in an organic solvent (not water) such as acetonitrile, methanol or ethanol. The drug is then dissolved with some kind of stabiliser in order to then be rapidly frozen. As a result of this procedure we obtain non-crystalline pharmaceutical substances, named amorphous forms or amorphous morphology, which can be dissolved at much higher concentrations than their corresponding (water) crystalline forms.
dissolved in an organic solvent (not water) such as acetonitrile, methanol or ethanol. The drug is then dissolved with some kind of stabiliser in order to then be rapidly frozen. As a result of this procedure we obtain non-crystalline pharmaceutical substances, named amorphous forms or amorphous morphology, which can be dissolved at much higher concentrations than their corresponding (water) crystalline forms. Thus ‘super-saturation’ of an aqueous solution is achieved, which facilitates the drug’s dissolution at high concentrations in the stomach and in the upper small intestine to be subsequently absorbed and distributed through the whole organism.
| Properties of crystalline and amorphous drugs | |
| Crystalline state | Amorphous state |
| Long range translational, rotational and conformational order | May exhibit short range order |
| Well defined melting point | Glass transition point[2] |
| Good flow properties[3] | Poor flow properties |
| More stable | Less stable |
| Less hygroscopic[4] | More hygroscopic |
| Relatively less soluble | Relatively more soluble |
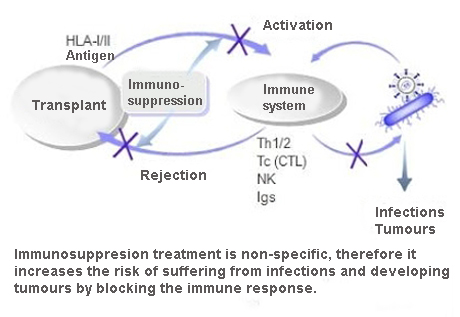
An application example of this type of particle engineering system has been developed by members of the medical school of the University of Texas in San Antonio and the Veterans Administration hospital, looking at a new administration route for immune-suppressing drugs.
This sort of medication prevents the rejection of transplanted organs, but at present, by being administered orally it can cause a series of undesirable side effects, like the development of different types of cancer.
The objective of the technologies described above was to administer a lower dosage of these drugs with accuracy to the site where its immune-suppressing action was necessary. In order to achieve this, prototypes of a new inhaled immune-suppressing drug were developed during a period of four to five years. The inhalation route of administration was selected because the patients who had undergone a lung transplant were the research target, and consequently the lungs were the aim of this medication.
Several tests were performed in mammals (mice and rats) where the product safety was proven. Low levels of the drug in the blood but high concentrations in the lungs were identified, which shows the validity of the inhalation route.
Then, the drug was tested in a small group of healthy human volunteers, under strict safety rules, and the results were similar to those in animals. Yet, this medication is still under development.
Several tests were performed in mammals (mice and rats) where the product safety was proven. Low levels of the drug in the blood but high concentrations in the lungs were identified, which shows the validity of the inhalation route.
Then, the drug was tested in a small group of healthy human volunteers, under strict safety rules, and the results were similar to those in animals. Yet, this medication is still under development.
In conclusion, the goal is the research of new routes of drug administration that are not available presently to benefit the patients by minimising side effects and reducing the systemic drug exposure[5].
[1] Action exerted by the drug once is absorbed and distributed by the bloodstream.
[2] Temperature at which an amorphous solid becomes soft and flexible upon heating or rigid and brittle upon cooling.
[3] These properties indicate a higher or lower fluidity of particles depending on their size, shape, absorbed moisture and density.
[4] Substances that absorb moisture from the surrounding environment.
[5] A type of exposure where, in contrast with topical or local exposure drugs, the entire organism is affected by a medication performance.
[2] Temperature at which an amorphous solid becomes soft and flexible upon heating or rigid and brittle upon cooling.
[3] These properties indicate a higher or lower fluidity of particles depending on their size, shape, absorbed moisture and density.
[4] Substances that absorb moisture from the surrounding environment.
[5] A type of exposure where, in contrast with topical or local exposure drugs, the entire organism is affected by a medication performance.
Sources: UTAustinX: UT.4.01x Take Your Medicine - The Impact of Drug Development.
http://www.slideshare.net/rcdreddi/limerick-meet-and-greet-ac-edited
http://chemistry.about.com/od/chemistryglossary/a/glasstransition.htm
https://docs.google.com/document/d/1TOwPCUSItCGOnUivKC_5kuTGw7kh9D5DPIK2U-V7D2A/edit#heading=h.90pmyxnddx1b
http://trasplante-de-organos.blogspot.com.es/2011/09/inmunologia-del-trasplante-la.html
http://www.slideshare.net/rcdreddi/limerick-meet-and-greet-ac-edited
http://chemistry.about.com/od/chemistryglossary/a/glasstransition.htm
https://docs.google.com/document/d/1TOwPCUSItCGOnUivKC_5kuTGw7kh9D5DPIK2U-V7D2A/edit#heading=h.90pmyxnddx1b
http://trasplante-de-organos.blogspot.com.es/2011/09/inmunologia-del-trasplante-la.html





Your opinion matters