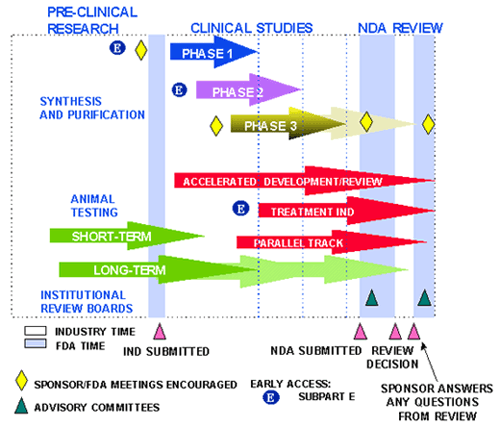
In the post titled: ‘Safety and efficacy of drugs’, we saw how the regulatory agency in the USA, the FDA (Food and Drug Administration), started the drug review process by submiting an Investigational New Drug (IND) application to request the start of clinical trials in humans.
Later, there is a review at two levels:
▣ A review at national level, where the FDA inspects all the pre-clinic work performed in the laboratory and the data set that led to the submission of that IND.
▣ A second review at local level (cities, towns, health systems) that is carried out by local entities named Institutional Review Boards (IRBs) regulated by the FDA. Their functions are to evaluate the number of patients that take part in the study, the study safety and its design.
After these assessments, if it is concluded that the benefits outweigh the risks the FDA and IRBs approve human trials.
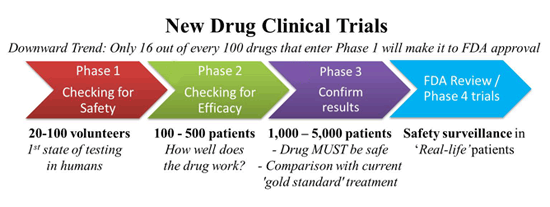
Clinical trials in humans are divided into three different phases:
▣ Phase I: they are small tests that involve from 10 to 20 healthy human volunteers, who do not have the disease of interest.
In this phase, the drug is, for the first time, administered to human subjects.
The objective of these studies is to find the tolerability and safety of the new product, as well as its pharmacokinetics[1].
In this phase, the drug is, for the first time, administered to human subjects.
The objective of these studies is to find the tolerability and safety of the new product, as well as its pharmacokinetics[1].
▣ Phase II: once the phase I has been completed without issue, the researchers proceed to the phase II where they determine, by comparison, if the new drug works better than an inactive product (placebo) or, at least, as well as another current drug.
To achieve this, half of the patients of the study are administered with the new drug and the other half with the inactive product or the drug already on the market.
Patients receive a wide range of doses of the drug with the purpose of finding out which one provides fewer side effects along with the best efficacy.
This phase involves between 100 and 500 test subjects.
To achieve this, half of the patients of the study are administered with the new drug and the other half with the inactive product or the drug already on the market.
Patients receive a wide range of doses of the drug with the purpose of finding out which one provides fewer side effects along with the best efficacy.
This phase involves between 100 and 500 test subjects.
▣ Phase III: if everything goes according to plan, we reach phase III where the new drug is given to thousands of patients. Once again, it is compared to a placebo or a medicine that already works to discover whether the new product is safe and efficient, this time at a larger scale.
In this phase, the tests occur in many centres not only in the US but also all over the world.
Another characteristic feature of phase III is that the drug is administered by healthcare providers or practising clinicians, which gives the FDA new data about the safety and effectiveness of the new medicine in the real environment of health care.
In this phase, the tests occur in many centres not only in the US but also all over the world.
Another characteristic feature of phase III is that the drug is administered by healthcare providers or practising clinicians, which gives the FDA new data about the safety and effectiveness of the new medicine in the real environment of health care.
In phases I, II, III and even subsequently, the safety of the new compound is always evaluated and is the main concern in these studies.
Biopharmaceutical companies do not conduct these clinical research studies alone, but working very closely with the FDA, in such a way that the FDA can halt a study at any moment, even before beginning, when it suspects that the patient safety could be at risk.
Equally, the FDA can finish a trial ahead of schedule when the drug is so effective that expectations are exceeded, so that the new medicine starts to be commercialised for those people who need it.
Equally, the FDA can finish a trial ahead of schedule when the drug is so effective that expectations are exceeded, so that the new medicine starts to be commercialised for those people who need it.
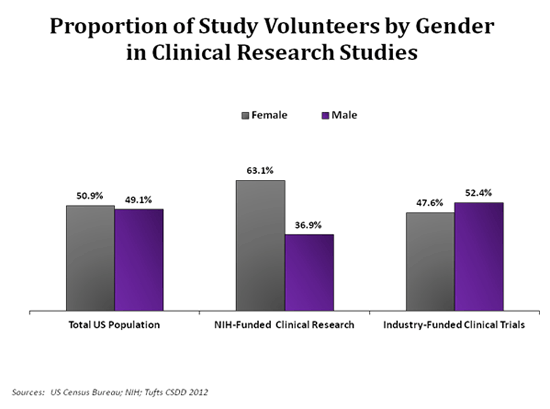
At the end of these clinical trials, a new application, named a New Drug Application (NDA), is drawn up and submitted to the FDA where it is examined by several members with different backgrounds: scientists, statisticians, clinicians… in order to give recommendations (as long as the drug fulfils the safety and effectiveness criteria).
The FDA also examines the facilities where the new drug will be manufactured in a standardised and robust way and confirms that they adhere to good manufacturing practices (GMP).
The FDA also reviews the set of information, called product or package labelling, distributed with the product, which helps patients and health providers to determine if a specific medicine is the most appropriate to treat a certain illness.
This leaflet includes information about the clinical testing done, the most suitable dosage, warnings about its safety (if any) and side effects.
This leaflet includes information about the clinical testing done, the most suitable dosage, warnings about its safety (if any) and side effects.
Lastly, the FDA decides whether the new medication is approved or not. If the result is negative, it can ask for further studies from the manufacturer or deny its use in the US permanently in the event that there is enough evidence about the drug’s ineffectiveness or doubts about its safety.
[1] Measurement of drug levels in the organism and how the drug is excreted from it.
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://www.ice-epilepsy.org/us-drug-approval-process.html
http://mscreations.org/clinical-trial-phases/
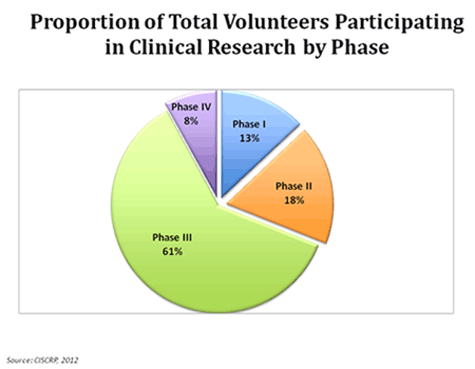
https://www.ciscrp.org/education-center/charts-and-statistics/during-participation/
http://www.ice-epilepsy.org/us-drug-approval-process.html
http://mscreations.org/clinical-trial-phases/
https://www.ciscrp.org/education-center/charts-and-statistics/during-participation/





Your opinion matters