Most people are more than familiar with the first type of biological macromolecules that I will explain: carbohydrates.
Carbohydrates are an essential part of our diet; they provide energy (4.3 Kcal/g) mainly through glucose. Among the rich natural sources of carbohydrates we find: vegetables, fruits and cereals. Their molecular structure is represented by the stoichiometric formula (CH2O)n, where “n” indicates the number of carbon atoms in the molecule. Therefore, in carbohydrate molecules the ratio C, H, O is always 1:2:1.Carbohydrates are divided into three types: monosaccharides, disaccharides, and polysaccharides.
Monosaccharides
Monosaccharides (mono-: one; sacchar-: sweet) are simple sugars; glucose being the most common among them. Most of them are named by the suffix: “-ose”. They contain a number of carbon atoms that vary from three to seven. Thus, we find trioses (three carbons), pentoses (five carbons) or hexoses (six carbons).
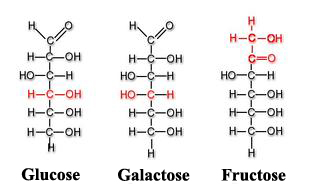
Monosaccharides are molecules that can adopt both linear chain shapes and ring shapes; these ring forms are frequently found in aqueous solutions. If monosaccharides contain an aldehyde group[1], the monosaccharide is known as aldose, however if the functional group is a ketone group[1], the monosaccharide is called ketose.
Monosaccharides are molecules that can adopt both linear chain shapes and ring shapes; these ring forms are frequently found in aqueous solutions. If monosaccharides contain an aldehyde group[1], the monosaccharide is known as aldose, however if the functional group is a ketone group[1], the monosaccharide is called ketose.
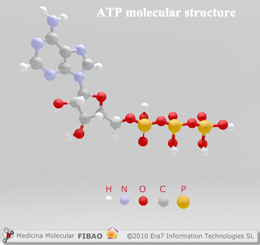
The monosaccharide glucose (C6H12O6) is one of the basic energy sources for the human being. During cell respiration, glucose releases energy which is used to produce ATP (adenosine triphosphate)[2]. Plants synthesise glucose using CO2 and water, their surpluses are stored/accumulated as starch which is catabolised (broken down by cells) when it is consumed by the human being or herbivorous animals.
Among other monosaccharides, we can also point out galactose (which is part of lactose or milk sugar) or fructose (present in fruits and honey). Both glucose, galactose and fructose[3] are examples of isomeric monosaccharides because they have the same chemical structure but they differ structurally and chemically due to the different arrangements of their functional groups around their assymetric (or chiral) carbons[4]. Glucose and galactose belong to the group that we saw previously called aldoses and fructose belongs to the ketose group.
Disaccharides
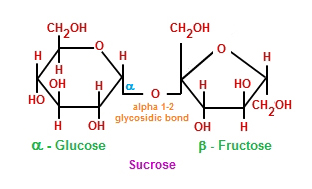
Disaccharides (di-: two) are formed when two monosaccharides undergo a dehydration synthesis. During this process the hydrogen atom of one monosaccharide is combined with the hydroxyl group of other monosaccharide, forming a covalent bond between them and releasing a water molecule. This covalent bond is named glycosidic bond, which is classified in two types: α and β, depending on if the hydroxyl group is located above or below the chiral carbon (also known as asymmetric carbon that we saw previously).
Among the most common disaccharides are found lactose, which is the result of the union of galactose and fructose. Milk is a natural source where lactose is found. Another example of disaccharides is maltose, or malt sugar, which is formed during a dehydration reaction performed by two glucose molecules. But, without a doubt, the most common disaccharide is sacarose, or table sugar, made up of fructose and glucose monomers.
Polysaccharides
Polysaccharides (poly-: many) are long chains of monosaccharides joined by glycosidic bonds. These chains can be branched or unbranched, containing different types of monosaccharides.
Among the main polysaccharides are: cellulose, glucogen, starch and chitin.
Among the main polysaccharides are: cellulose, glucogen, starch and chitin.
Starch
As I mentioned earlier, plants are capable of synthesising glucose from water and CO2. When glucose production exceeds the energy needs of the plant, the surpluses/excesses are stored as starch in different parts of the plant like the root and seeds, providing feed during their germination.
When starch is consumed by animals or humans in their diets, it is first broken down by enzymes (such as salivary amylases) into smaller molecules like glucose, before being absorbed by cells to satisfy their energy needs.
When starch is consumed by animals or humans in their diets, it is first broken down by enzymes (such as salivary amylases) into smaller molecules like glucose, before being absorbed by cells to satisfy their energy needs.
Glucogen
Glucogen, composed of glucose monomers, is how glucose is stored in humans and other vertebrates. It is the equivalent to starch in plants and it is accumulated in muscular cells and the liver. When blood glucose levels decrease, these glucose deposits are demanded and glucogen is broken down into glucose during a process called glycogenolysis.
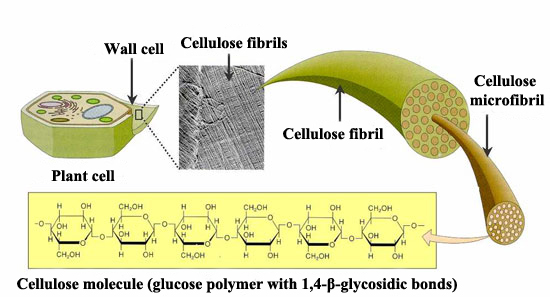
Cellulose
Cellulose, also made up of glucose monomers, is the most abundant polymer. It is the main element of plant cell walls, giving them structural support. In cellulose the basic monomers are tightly packed, giving cellulose its typical fibrous structure which confers stiffness and high tensile strength, i.e. structural support for plants.
Unlike human beings, herbivores have bacteria and protists[5] inside their digestive system which segregate the cellulase enzyme to help digest the cellulose they consume in their grass. By the action of this enzyme, cellulose is broken down into glucose monomers and in this way it can be used as energy source.
Unlike human beings, herbivores have bacteria and protists[5] inside their digestive system which segregate the cellulase enzyme to help digest the cellulose they consume in their grass. By the action of this enzyme, cellulose is broken down into glucose monomers and in this way it can be used as energy source.
Chitin
The fourth polysaccharide to point out is chitin, which is the main component in fungi cell walls and the exoskeleton (external skeleton) of arthropods, which protects their internal organs. Chitin is a polysaccharide with a high nitrogen content, constituted by repetitive units of N-acetyl-β-glucosamine, a kind of modified sugar.
BENEFITS OF CARBOHYDRATES
Carbohydrates are composed of both soluble and insoluble elements. Among the insoluble carbohydrates we find fiber, which is mainly cellulose. Cellulose helps to regulate the intestinal transit and the glucose consumption rate. It also binds to the blood cholesterol in the small intestine and in this way the cholesterol is eliminated by being excreted from the organism in faeces. Also, diets high in fibre can help to protect against colorectal cancer.
Glucose is provided by the carbohydrates that we consume. Once it is broken down during cell respiration, glucose produces ATP molecules, known as the energy-currency of cells, providing fast energy for any organism.
Consequently, without the consumption of carbohydrates, the availability of instant energy for the body (or any other organism) would be deeply reduced.
Consequently, without the consumption of carbohydrates, the availability of instant energy for the body (or any other organism) would be deeply reduced.
For all these reasons, eliminating the ingestion of carbohydrates, like some hypocaloric diets recommend in order to achieve a fast weight loss, is questionably healthy. To accomplish such an aim, it would be more healthy to balance appropriate low GI carbohydrates present in vegetables, fruits and cereals with a balanced level of proteins, vitamins and lipids, a suitable water intake and doing exercise appropriate to our age, gender and physical fitness.
[1] Both are organic compounds characterised by containing the carbonyl functional group (C=O). But aldehydes contain the carbonyl group bonded to at least one hydrogen atom, whereas ketones contain the carbonyl group bonded to two carbon atoms.
[2] Molecule used by all living organism to provide energy in chemical reactions. It is considered as the “energy-currency” of the metabolism.
[3] They all are hexoses: six carbon atoms in their structures.
[4] Carbon atom attached to four different types of atoms or group of atoms.
[5] Eukaryotic (their cell nucleus is delimited by a membrane) organisms, usually unicellular, which due to their own characteristics cannot be included in the rest of the kingdoms (animals, plants and fungi) within this category.
[2] Molecule used by all living organism to provide energy in chemical reactions. It is considered as the “energy-currency” of the metabolism.
[3] They all are hexoses: six carbon atoms in their structures.
[4] Carbon atom attached to four different types of atoms or group of atoms.
[5] Eukaryotic (their cell nucleus is delimited by a membrane) organisms, usually unicellular, which due to their own characteristics cannot be included in the rest of the kingdoms (animals, plants and fungi) within this category.
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://medmol.es/glosario/121012glosariomedmol_atp/
https://kel-tay-lii.wikispaces.com/A.+Intro+to+Phys
http://chemistry.tutorvista.com/organic-chemistry/oligosaccharides.html
http://www.entrenasalud.es/glucogeno-y-deporte-un-gran-deposito-de-energia/
http://www.asturnatura.com/articulos/envoltura-celular/pared-celular.php
http://medmol.es/glosario/121012glosariomedmol_atp/
https://kel-tay-lii.wikispaces.com/A.+Intro+to+Phys
http://chemistry.tutorvista.com/organic-chemistry/oligosaccharides.html
http://www.entrenasalud.es/glucogeno-y-deporte-un-gran-deposito-de-energia/
http://www.asturnatura.com/articulos/envoltura-celular/pared-celular.php






Your opinion matters