Lipids are a type of hydrocarbon mostly made up of nonpolar C-C and C-H bonds, which makes them hydrophobic (they repel water) or insoluble in water.
Lipids (more commonly known as fats) constitute a basic element in many hormones, as well as in cell membranes. They are an important source of long-term fuel for cells, they provide heat insulation for both animals and plants and they form a protective hydrophobic outer layer over fur or feathers of aquatic birds and mammals.
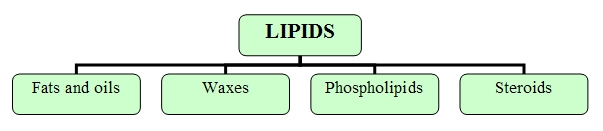
Within lipids we find the following categories:Lipids (more commonly known as fats) constitute a basic element in many hormones, as well as in cell membranes. They are an important source of long-term fuel for cells, they provide heat insulation for both animals and plants and they form a protective hydrophobic outer layer over fur or feathers of aquatic birds and mammals.
Fats and oils
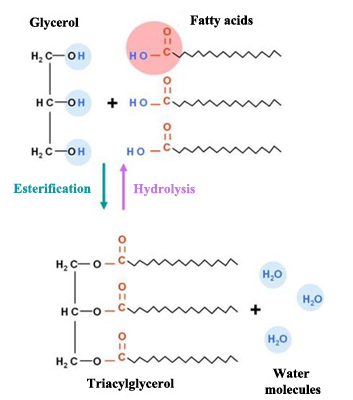
Fats, due to their chemical structure, are also named triacylglycerols or triglycerides. They are made up of two basic components: glycerol and fatty acids.
Glycerol is an organic component (specifically, a kind of alcohol) with three carbon atoms, five hydrogen atoms and three hydroxyl groups (OH). Fatty acids, in turn, are composed of a long chain of hydrocarbons to which a carboxyl group is attached.
The number of carbon atoms in fatty acids generally vary between four and thirty-six, although the most common contain 12 to 18 carbons. Fatty acids’ three carbon atoms (in the glycerol molecule) are joined by an ester bond and an oxygen atom. During the formation of this bond three water molecules are released.
Glycerol is an organic component (specifically, a kind of alcohol) with three carbon atoms, five hydrogen atoms and three hydroxyl groups (OH). Fatty acids, in turn, are composed of a long chain of hydrocarbons to which a carboxyl group is attached.
The number of carbon atoms in fatty acids generally vary between four and thirty-six, although the most common contain 12 to 18 carbons. Fatty acids’ three carbon atoms (in the glycerol molecule) are joined by an ester bond and an oxygen atom. During the formation of this bond three water molecules are released.
Fatty acids are divided into two groups: saturated and unsaturated.
The first only have simple bonds between the adjacent carbons of the hydrocarbon chain. An example is the stearic acid formed by 18 carbon atoms and in which the number of hydrogen atoms attached to the carbon backbone is the maximum (no more hydrogen atoms can be attached). This acid is present in oils and in animal and plant fats. Among its uses we find the manufacturing of soaps, candles, plastics, cosmetics and as a softening agent for rubber.
Unsaturated fatty acids, on the contrary, have double bonds between their carbon atoms. Among the most common unsaturated fatty acids we find oleic acid, known for its beneficial impact on blood vessels, thus decreasing the risk of liver and cardiovascular diseases.
Unsaturated fatty acids are popularly known as oils, which are liquid at room temperature and can be monounsaturated when they have one double carbon bond in the molecule (olive oil) or polyunsaturated if they have more than one (canola oil).
The first only have simple bonds between the adjacent carbons of the hydrocarbon chain. An example is the stearic acid formed by 18 carbon atoms and in which the number of hydrogen atoms attached to the carbon backbone is the maximum (no more hydrogen atoms can be attached). This acid is present in oils and in animal and plant fats. Among its uses we find the manufacturing of soaps, candles, plastics, cosmetics and as a softening agent for rubber.
Unsaturated fatty acids, on the contrary, have double bonds between their carbon atoms. Among the most common unsaturated fatty acids we find oleic acid, known for its beneficial impact on blood vessels, thus decreasing the risk of liver and cardiovascular diseases.
Unsaturated fatty acids are popularly known as oils, which are liquid at room temperature and can be monounsaturated when they have one double carbon bond in the molecule (olive oil) or polyunsaturated if they have more than one (canola oil).
Plants store fats or oils in their seeds, which are used as energy deposits during their development. In mammals, however, fat constitute globules, which take up most of the volume of a very specialised kind of cell called an ‘adipocyte’.
Unsaturated fats or oils have usually a plant origin and contain cis unsaturated fatty acids (visit the entry “The carbon atom” to remember the concepts cis and trans configuration). This cis double bond causes a bend or “twist” which prevent fatty acids from being compacted, keeping them in liquid state at room temperature. As examples of unsaturated fats: olive, corn, canola and cod oils. These kinds of fats help us to reduce our cholesterol levels (in contrast to saturated fats which contribute to the formation of plaques in our arteries).
Trans fats
Trans fats are present in some food products like margarine or peanut butter and can lead to increased levels of low density lipoproteins (LDL), more commonly known as “bad” cholesterol, which is deposited on arteries walls as plaque, producing cardiovascular disorders.
These trans fats are manufactured by the food industry by a method called ‘hydrogenation’, in which the double bonds of the cis structure of the hydrocarbon chain become double bonds in the trans configuration. During this process, oils are solidified by injecting gaseous hydrogen into them, so that they can acquire that desirable semisolid consistency in many processed foods.
These trans fats are manufactured by the food industry by a method called ‘hydrogenation’, in which the double bonds of the cis structure of the hydrocarbon chain become double bonds in the trans configuration. During this process, oils are solidified by injecting gaseous hydrogen into them, so that they can acquire that desirable semisolid consistency in many processed foods.
Omega fatty acids
Both omega-3 fatty acids and omega-6 fatty acids belong to the group of the essential fatty acids that our organism requires but is not able to synthesise; thus we must ingest them through our diet.
The terms omega-3 and omega-6 indicate that the third and the sixth carbon, counting from the far end of the hydrocarbon chain, are the ones that are attached to their adjacent carbon by a double bond.
Among the food sources of omega-3 we find some oily fish like trout, tuna and salmon. These types of fatty acids reduce blood pressure and the triglyceride levels in blood, they help to prevent thrombosis, heart attacks and may help to decrease the risk of certain kinds of cancer.
The terms omega-3 and omega-6 indicate that the third and the sixth carbon, counting from the far end of the hydrocarbon chain, are the ones that are attached to their adjacent carbon by a double bond.
Among the food sources of omega-3 we find some oily fish like trout, tuna and salmon. These types of fatty acids reduce blood pressure and the triglyceride levels in blood, they help to prevent thrombosis, heart attacks and may help to decrease the risk of certain kinds of cancer.
Fats are not only excellent energy deposits in the long term and provide isolation for the body, but also enable us to digest fat-soluble vitamins. Therefore, despite the bad publicity that they have received, the “healthy” fats must be consumed in balanced diets regularly.
Waxes
Waxes are comprised long chains of fatty acids esterified[1] to long-chain alcohol.
Because of their hydrophobic nature, they perform a protective function on the outer coating of the leaves of some plants and the feathers of aquatic birds.
Because of their hydrophobic nature, they perform a protective function on the outer coating of the leaves of some plants and the feathers of aquatic birds.
Phospholipids
Phospholipids are formed by fatty acid chains attached to a glycerol or sphingosine[2] backbone, where two fatty acids make up a diacylglycerol molecule and the third carbon of the glycerol backbone is occupied by a modified phosphate group.
The compound formed by the diacylglycerol molecule and the phosphate group constitute the phosphatidates, which are the precursors of phospholipids.
Phospholipids are part of the outer layer of animal cells and the main element of plasma membranes, which play a fundamental role in the cellular communication.
The compound formed by the diacylglycerol molecule and the phosphate group constitute the phosphatidates, which are the precursors of phospholipids.
Phospholipids are part of the outer layer of animal cells and the main element of plasma membranes, which play a fundamental role in the cellular communication.
Phospholipids make up these membranes in such a way that the phospholipid tails (which are hydrophobic fatty acids that cannot interact with water) face internally and the phospholipid head (which is the hydrophilic phosphate group that interacts with water) faces externally, in contact with the aqueous environment. By having a hydrophobic and a hydrophilic part, phospholipids are classified as amphiphatic molecules.
The dynamic nature of plasma membranes is due to their being formed by phospholipids. In contact with water, phospholipid molecules are ordered spontaneously in a spherical structure called a micelle, with the heads (polar) facing the outside and the tails (nonpolar) facing the inside of these structures, just like plasma membranes.
Steroides
In spite of the fact that steroids are not very similar to the rest of the lipids, on account of the fact that they have a fused ring structure, they are included within this category as they are insoluble in water.
All steroids are made up of four carbon rings and many of them also have the –OH functional group, which allows them to be classified as alcohols (sterols).
All steroids are made up of four carbon rings and many of them also have the –OH functional group, which allows them to be classified as alcohols (sterols).
In addition, some of them, like the cholesterol molecule, have a short hydrocarbon tail. Cholesterol is the most common steroid in the human being and animals, it is synthesised in the liver and is the precursor to vitamin D and also of bile salts, which help to metabolise the fats we ingest to be absorbed by cells afterwards.
Cholesterol is secreted by the endocrine glands and the gonads, as it is the precursor of steroid hormones like estradiol[3] and testosterone. Therefore, despite the bad name that cholesterol has among lay people, this molecule plays a vital role for the proper functioning of our organism.
Cholesterol is secreted by the endocrine glands and the gonads, as it is the precursor of steroid hormones like estradiol[3] and testosterone. Therefore, despite the bad name that cholesterol has among lay people, this molecule plays a vital role for the proper functioning of our organism.
[1] Esterification reactions are those by an ester is obtained, usually from the reaction of a carboxylic acid and an alcohol.
Esters are organic compounds made by substituting an acid by an alkyl or other organic group.
[2] Amino alcohol made up of 18 carbons, forming an unsaturated hydrocarbon chain.
[3] Female sex hormone.
Esters are organic compounds made by substituting an acid by an alkyl or other organic group.
[2] Amino alcohol made up of 18 carbons, forming an unsaturated hydrocarbon chain.
[3] Female sex hormone.
Sources: OpenStax College, Biology. OpenStax College. 30 May 2013.
http://www.genomasur.com/BCH/BCH_libro/capitulo_02.htm
http://www.salud180.com/sustancias/acido-estearico
http://herbolaria.wikia.com/wiki/%C3%81cido_oleico
https://www.flickr.com/photos/thame/3302072732
http://www.eufic.org/article/es/artid/La-importancia-de-los-acidos-grasos-omega-3-y-omega-6/
http://www.uhu.es/08007/documentos%20de%20texto/apuntes/2005/pdf/tema_03_lipidos.pdf
http://www.fisicanet.com.ar/biologia/introduccion_biologia/ap11_lipidos.php
http://brainly.com.br/tarefa/453268
http://www.calpoly.edu/~jfernsle/Research/Biophysics/BiophysResearch.html
http://www.genomasur.com/BCH/BCH_libro/capitulo_02.htm
http://www.salud180.com/sustancias/acido-estearico
http://herbolaria.wikia.com/wiki/%C3%81cido_oleico
https://www.flickr.com/photos/thame/3302072732
http://www.eufic.org/article/es/artid/La-importancia-de-los-acidos-grasos-omega-3-y-omega-6/
http://www.uhu.es/08007/documentos%20de%20texto/apuntes/2005/pdf/tema_03_lipidos.pdf
http://www.fisicanet.com.ar/biologia/introduccion_biologia/ap11_lipidos.php
http://brainly.com.br/tarefa/453268
http://www.calpoly.edu/~jfernsle/Research/Biophysics/BiophysResearch.html












Your opinion matters