Delivering aerosols via inhalation has been practiced for millennia. As a matter of fact, since prehistory, where men began to use certain subtances with therapeutical and hallucinogenic purposes, such as cohoba (made from the beans of a mimosa species) or cannabis.
But aerosols only first replaced oral medication (tablets, pills, capsules…) in the 1950s for the treatment of respiratory diseases because oral medication caused severe side effects. The first meter-dose inhaler was made simply from a Coke bottle, some perfume valves and propellant systems.
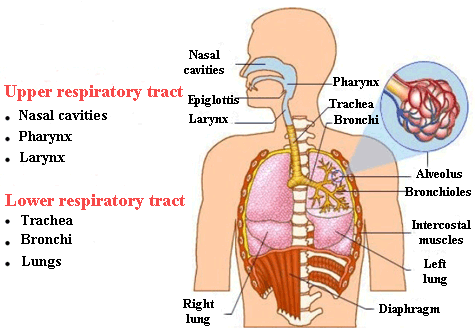
The main challenge that aerosols face to penetrate the lungs and the upper airways (mouth, pharynx, larynx and trachea) is that these organs are very well equipped to filter dust and other particles. Hence, the challenge consists of designing the aerosol particles in such a way that they do not end up in the mouth or throat as opposed to the lungs.
These particles must be produced with a size or aerodynamic diameter between 1 to 5 μm, in order to pass beyond the upper airways, which act as a filter. As you can imagine, manufacturing such tiny particles is a hugely complex task.
For instance, those microscopic particles which are made of dry powder, have a very large surface area, which makes them very sticky. Consequently, the major force that acts on them is Van der Waals interaction (“Chemical bonds”) and not gravity (e.g. when you write with a chalk on a chalkboard it stays on and does not fall off due to these interactions rather than gravity). So, the objective is to overcome these Van der Waals forces and develop methods of deaggregating aerosol particles.
These particles must be produced with a size or aerodynamic diameter between 1 to 5 μm, in order to pass beyond the upper airways, which act as a filter. As you can imagine, manufacturing such tiny particles is a hugely complex task.
For instance, those microscopic particles which are made of dry powder, have a very large surface area, which makes them very sticky. Consequently, the major force that acts on them is Van der Waals interaction (“Chemical bonds”) and not gravity (e.g. when you write with a chalk on a chalkboard it stays on and does not fall off due to these interactions rather than gravity). So, the objective is to overcome these Van der Waals forces and develop methods of deaggregating aerosol particles.
Lots of the aerosols used to treat pulmonary pathologies (e.g. cystic fibrosis, Chronic Obstructive Pulmonary Disease or COPD and asthma) are not really well designed for patient use.
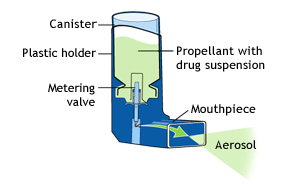
For example, metre-dose inhalers have been used successfully for many years, however a lot of patients, especially children and the elderly, cannot or do not use them correctly. These inhalers present a major difficulty for those with coordination issues in activating the actuation manoeuvre with the inhalation manoeuvre.
On the other hand, the dry powder inhalers that we find currently on the market are all all active, which means that the patient needs to provide all the necessary energy so that the aerosol gets into the lungs via the airways.
all active, which means that the patient needs to provide all the necessary energy so that the aerosol gets into the lungs via the airways.
The efficacy of these type of aerosols depends on how hard you inhale through the device. In this way, if you do not breath fast or strong enough, the aerosol particles will not be deaggregated enough to reach the lungs and they will mainly accumulate in the throat.
As a consequence, these sort of inhalers are not suitable for those patients who do not have proper lung function.
 all active, which means that the patient needs to provide all the necessary energy so that the aerosol gets into the lungs via the airways.
all active, which means that the patient needs to provide all the necessary energy so that the aerosol gets into the lungs via the airways. The efficacy of these type of aerosols depends on how hard you inhale through the device. In this way, if you do not breath fast or strong enough, the aerosol particles will not be deaggregated enough to reach the lungs and they will mainly accumulate in the throat.
As a consequence, these sort of inhalers are not suitable for those patients who do not have proper lung function.
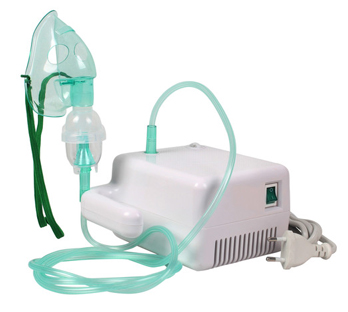
A third type of inhaler is a nebulizer or water-based nebulizer spray systems, usually bulky units, not very portable, used at home or in the clinic, although some technical developments have been made lately to make them more portable and battery operated.
So, these are the different devices for the treatment of respiratory diseases by the inhalation route and their selection is based upon the patient’s needs and the kind of drug to be delivered.
There is an interesting story about the formulation of the meter-dose inhalers. At the beginning, when these devices were invented, back in the 1950s, they used chlorofluorocarbon (CFCs) propellants into which the drug was suspended or dissolved. But, due to reports in the 1970s about ozone layer depletion because of the use of these propellant gases, and their subsequent ban in the late eighties, the pharmaceutical industry replaced them with hydrofluroalkanes (HFAs) with similar chemical and physical properties to the CFCs.
In spite of comparable properties, the HFAs behaved in a very different way with the pharmacological molecules and the rest of excipients. For example, some surfactants[1] used in drug formulation, like oleic acid, were soluble in the CFCs but not in the HFAs.
Because of this pharmaceutical companies adopted several strategies to reformulate the same drug into these propellants gases.
A meaningful example is the product called QVAR[2], in which the drug is dissolved, using a co-solvent (diluent), specifically ethanol, together with hydrofluroalkane propellants. And it turned out that the aerosol produced is a much finer mist with a much finer droplet size. In this way, the amount of aerosol particles deposited in mouth and throat decreased tremendously, but, on the other hand, there was a dramatic increase in lung delivery.
This new approach provided a competitive advantage for the company that created it in comparison to the competition, which tried to adapt the performance of the HFA propellants in a similar way to the older-style CFCs.
A meaningful example is the product called QVAR[2], in which the drug is dissolved, using a co-solvent (diluent), specifically ethanol, together with hydrofluroalkane propellants. And it turned out that the aerosol produced is a much finer mist with a much finer droplet size. In this way, the amount of aerosol particles deposited in mouth and throat decreased tremendously, but, on the other hand, there was a dramatic increase in lung delivery.
This new approach provided a competitive advantage for the company that created it in comparison to the competition, which tried to adapt the performance of the HFA propellants in a similar way to the older-style CFCs.
[1] Substance that act as a detergent, emulsifier or humectant and allows to reduce the surface tension in a fluid.
[2] Anti-inflammatory inhaler indicated for the treatment of asthma attacks.
[2] Anti-inflammatory inhaler indicated for the treatment of asthma attacks.
Sources: UTAustinX: UT.4.01x Take Your Medicine - The Impact of Drug Development.
http://www.telegraph.co.uk/news/newstopics/howaboutthat/3225729/Stone-Age-man-took-drugs-say-scientists.html
http://es.slideshare.net/CAWIMECA/el-sistema-respiratorio-42882484
http://blogs.20minutos.es/yaestaellistoquetodolosabe/tag/clorofluorocarbonos/
http://definicion.de/surfactante/
http://www.asthma.ca/adults/treatment/meteredDoseInhaler.php
http://es.slideshare.net/luciagorreto/taller-asma-2015-inhaladores
http://www.drtrust.in/products/nebuliser-machine
http://www.telegraph.co.uk/news/newstopics/howaboutthat/3225729/Stone-Age-man-took-drugs-say-scientists.html
http://es.slideshare.net/CAWIMECA/el-sistema-respiratorio-42882484
http://blogs.20minutos.es/yaestaellistoquetodolosabe/tag/clorofluorocarbonos/
http://definicion.de/surfactante/
http://www.asthma.ca/adults/treatment/meteredDoseInhaler.php
http://es.slideshare.net/luciagorreto/taller-asma-2015-inhaladores
http://www.drtrust.in/products/nebuliser-machine





Your opinion matters